My name is Lynsay Hunter and I am the newest recruit to the MHRA laboratories inspection team, having previously worked in a variety of laboratory and quality assurance roles. My first MHRA event was the annual laboratories symposium held in …
The 2017 Orange and Green Guides are almost ready for publication.
Today sees the launch of the MHRA Blood Forum
The annual GCP Symposium was held by the MHRA on 20 September 2016 in Birmingham and was repeated on the 21 September 2016.
In October 2015 the MHRA was audited under the Joint Audit Programme (JAP) for EEA GMP Inspectorates.
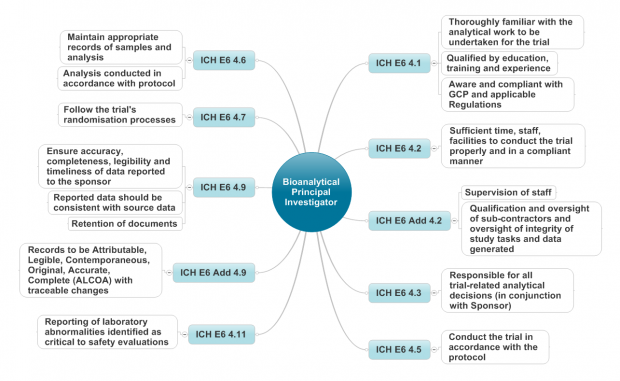
Earlier this year I represented the MHRA at the 10th Workshop on Recent Issues in Bioanalysis (WRIB).
Over the past few years we have seen our events grow in popularity; we have gone from 6 a year to over 30!
When I applied to join the Inspectorate I was working as a Qualified Person. I’d been on the receiving end of many inspections and thought that the role looked to be a hugely interesting one with a great amount of …
The MHRA GMDP Inspection Teams work closely with the Veterinary Medicines Directorate (VMD) Inspection Teams to ensure that manufacturers and distributors of Veterinary Medicinal Products (VMPs) are inspected appropriately and to the correct standards.
In July 2003 the Medicines and Healthcare products Regulatory Agency (MHRA) introduced a statutory pharmacovigilance inspection programme of marketing authorisation holders (MAHs) in the United Kingdom. The revisions to the pharmacovigilance legislation implemented in 2012 are supportive of the inspection of …