Exports and customs procedures

Those of you who attended our symposiums in either Glasgow or London during December 2016 will have heard Jane Sewell – International Trade Development Liaison Officer from HMRC speak about the Unified Customs Code.

Those of you who attended our symposiums in either Glasgow or London during December 2016 will have heard Jane Sewell – International Trade Development Liaison Officer from HMRC speak about the Unified Customs Code.

At the GMDP Symposium 2016, Andrew Hopkins and I gave a presentation about the Inspection Action Group (IAG) and Compliance Management Team (CMT), to highlight the systems and processes in place for when compliance issues are identified at manufacturing and …

It was a case of “lights, camera, action!” for the GMP days of the 2016 MHRA Symposium, with speakers making use of video clips, music, and pictures in order to deliver key messages.

Hello and welcome to Reference Safety Information II. Hopefully you will all have read my first Reference Safety Information (RSI) post which focused on how the RSI should be Identifiable, Approved and Consistent.

My name is Lynsay Hunter and I am the newest recruit to the MHRA laboratories inspection team, having previously worked in a variety of laboratory and quality assurance roles. My first MHRA event was the annual laboratories symposium held in …

The annual GCP Symposium was held by the MHRA on 20 September 2016 in Birmingham and was repeated on the 21 September 2016.
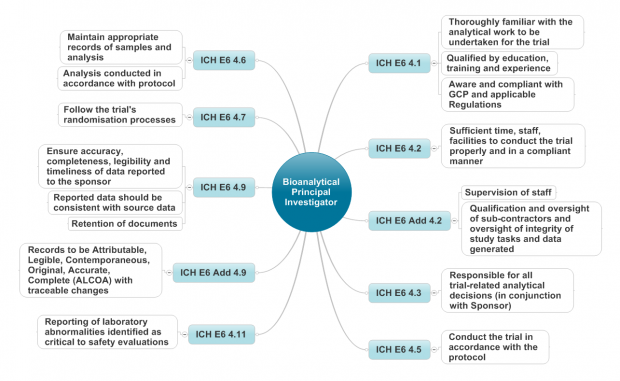
Earlier this year I represented the MHRA at the 10th Workshop on Recent Issues in Bioanalysis (WRIB).

Over the past few years we have seen our events grow in popularity; we have gone from 6 a year to over 30!

Just 4 weeks ago the MHRA hosted the PIC/S Committee meeting, PIC/S Executive Bureau meeting, and PIC/S Annual Seminar at the Museum of Science and Industry (MOSI) in Manchester.

On 22nd September 2016 the MHRA Inspectorate will be holding another GLP and GCP Laboratories Symposium.