It was a case of “lights, camera, action!” for the GMP days of the 2016 MHRA Symposium, with speakers making use of video clips, music, and pictures in order to deliver key messages.
As the GMDP Operations Manager with overall responsibility for planning and delivery of the event, I am pleased to be able to share with you some of the highlights of what was a professional and well received event.
The Inspectorate have taken to heart one of the MHRA’s priorities of supporting innovation and applied this to ensure the symposium was keeping up to date with emerging technologies. As well as a number of new presenters taking to the stage this year, the event made use of the MHRA Event App for the first time. The app enabled delegates to access presentation materials and to participate in the interactive sessions using their own electronic device.
The GMP day started with an opening presentation by Richard Andrews (Unit Manager Inspectorate Operations GMP and PV), who provided an update on applicable Agency, International and Inspectorate activities. This included confirmation that the 2017 editions of both the Orange Guide and Green Guide are on track to be published in January 2017 and also confirmation that no routine activities have been put on hold as a result of Brexit.
George Collins (GMDP Inspector) then took to the stage to provide a regulatory update, which covered recent changes now in effect, and on-going and planned updates to EU GMP, Q&As and guidance for industry. George played the role of anchor man in delivering the regulatory news and was supported by video pop-ups from subject matter experts, who provided more detailed update reports. The following diagram provides a timeline of past and future changes in the pipeline.
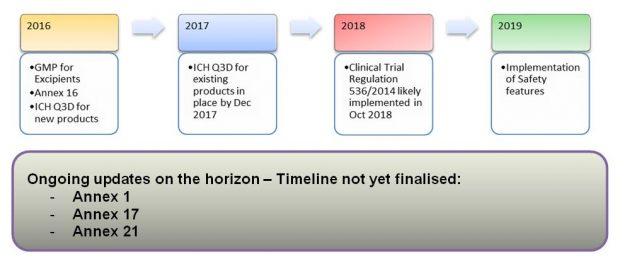
The take home message was that the structure of GMP is changing. The EU Commission has taken a new approach to publication of specialist guidance. Organisations that manufacture a combination of authorised products, IMPs and ATMPs will need to implement a quality system which accommodates the requirements of separate GMP guidances.
Mark Birse (Group Manager Inspectorate) then provided an update on current international coalition initiatives, which included updates on the various Pharmaceutical Inspection Co-operation Scheme (PIC/S) activities MHRA is leading and supporting, the International Coalition of Medicines Regulatory Agencies (ICMRA) and the EU/USA Mutual Reliance Initiative (MRI).
Mark explained that the ICMRA project aims to determine if it is feasible to take a risk-based approach to international inspections, placing reliance on data provided by the site and by their national regulator to carry out desktop assessments rather than inspecting the site. The phase 1 implementation has now been completed and phase 2 will be starting in Jan 2017 to expand the number of countries involved. The proposal longer-term is for the operational phase to be transferred to PIC/S.
The MRI is a strategic collaboration between EU regulatory authorities and the US-FDA to evaluate whether we have comparable regulatory and procedural frameworks for inspections of manufacturers of human medicines so that we can rely on each other’s information. The FDA is continuing to assess each EU regulator. The UK assessment has been finalised and the decision is now awaiting ratification. The current proposal is that the initiative will be 'signed off' in January 2017, and enter into operation later in 2017 on a voluntary basis for inspections carried out in member territories, with a view to making it a permanent requirement in 2019.
The agenda then moved to an interactive session taking another look at data integrity, and explored why we are still seeing stories in the media relating to data governance and data integrity failures at manufacturing sites, despite the publication of MHRA and international guidance for industry. This thought provoking session was presented by Graham Carroll (GMDP Inspector) and David Churchward (Expert GMDP Inspector), who concluded that effective implementation of data governance requires understanding of organisational behaviour, business process, data lifecycle, data risk, and critical thinking.
The next session provided an overview of the processes and possible outcomes of the Inspection Action Group (IAG) and Compliance Management Team (CMT) activities. Alan Moon (GMDP Inspector) and Andrew Hopkins (Expert GMDP Inspector) presented the session, which emphasised that the primary function of the two escalation processes is to protect the patient. An interactive poll of delegates revealed that over 70% were unaware of the CMT, which is a non-statutory process managed within the Inspectorate by Senior and Expert Inspectors, Operations and Unit Managers. The process was introduced by the GMP Inspectorate in late 2013/early 2014, with the aim to help direct companies back into a state of acceptable compliance, maintain supply of medicines, and avoid regulatory action.
Vivian Rowland (GMDP Inspector) then provided an update on deficiency data trending for 2015 and 2016 and presented examples from the most cited deficiency topic areas. To highlight one area of international collaboration the Inspectorate is involved with, Vivian provided some background to the work of the PIC/S working Group on Harmonisation of Classification of Deficiencies and the development of a tool for Inspectorates to improve harmonised risk classification of GMP deficiencies. It was reassuring to see that of the top ten most cited deficiencies across PIC/S countries, eight of the ten were the same as those within MHRA’s top ten of most citied deficiencies.
Quality Systems was the number one most cited deficiency by both MHRA and PIC/S countries, with a particular weakness noted in the investigation and reporting of quality incidents, deviations, customer complaints, out of specification investigations. It was therefore very timely that the next session on the agenda focused on the investigation of anomalies. Delegates were given the opportunity to benefit from some “tricks of the trade”, as Ewan Norton (GMDP Inspector) demonstrated some excellent practical tips on how to improve the effectiveness of investigations, to identify common causes of error and ensure effective CAPA is implemented.
This year’s event also featured two Q&A sessions which gave the audience the opportunity to pose Q&As to the MHRA panel. Delegates were invited to submit questions to the panel throughout the day using the MHRA Events App, and a wide range of questions were answered by the panel of Expert and Senior Inspectors. The Inspectorate commit to providing a written response to all questions submitted and the Q&As will be collated and made available to delegates in the New Year.
The event was drawn to a close by Richard Andrews who reminded delegates of the key themes of the day and take home messages which were:
- Organisations need to maintain awareness of GMP requirements and ensure there are systems in place to identify new requirements and implement the required changes within the pharmaceutical quality system
- Organisations need to consider the impact of company culture and management behaviour – the data integrity, IAG/CMT, deficiency data and investigation of anomalies presentations all identified company culture as being a key factor in improving GMP compliance
- MHRA has, and plans to maintain a key role in EU and International initiatives
As with the use of new technologies this year, we are keen to continue evolving the format and content of the symposium to enhance stakeholder engagement. We are interested in receiving post evaluation feedback from delegates, and in response to this blog, to help us plan for next year’s event. 2016 was the third year we have held a four day joint GMP and GDP event, and we would be interested to hear your thoughts on how the joint back to back event format works for you.
Don’t miss the next post, sign up to be notified by email when a new post is published on the Inspectorate blog.
Access our guidance on good practice for information on the inspection process and staying compliant.

