Good clinical practice
Global regulatory collaboration relating to data integrity remains a priority for MHRA and its international partners. In October 2016 I attended international data integrity workshops in China, hosted by CPAPE...
Hello and welcome to Reference Safety Information II. Hopefully you will all have read my first Reference Safety Information (RSI) post which focused on how the RSI should be Identifiable, Approved and Consistent.
In the October blog post we told you about the successful audit of the GMP Inspectorate under the JAP programme. Later that month the entire Inspectorate was assessed as part of the BEMA programme.
My name is Lynsay Hunter and I am the newest recruit to the MHRA laboratories inspection team, having previously worked in a variety of laboratory and quality assurance roles. My first MHRA event was the annual laboratories symposium held in …
The annual GCP Symposium was held by the MHRA on 20 September 2016 in Birmingham and was repeated on the 21 September 2016.
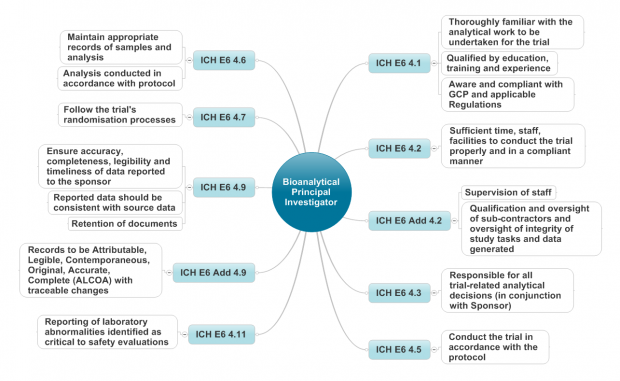
Earlier this year I represented the MHRA at the 10th Workshop on Recent Issues in Bioanalysis (WRIB).
Over the past few years we have seen our events grow in popularity; we have gone from 6 a year to over 30!
When I applied to join the Inspectorate I was working as a Qualified Person. I’d been on the receiving end of many inspections and thought that the role looked to be a hugely interesting one with a great amount of …
Just 4 weeks ago the MHRA hosted the PIC/S Committee meeting, PIC/S Executive Bureau meeting, and PIC/S Annual Seminar at the Museum of Science and Industry (MOSI) in Manchester.
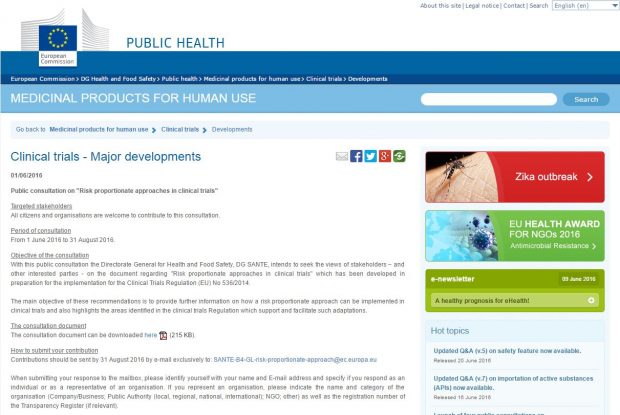
There are currently four consultations on guidance documents that have been developed in preparation for the implementation of the Clinical Trial Regulation (EU) No 536/2014. The MHRA GCP Inspectorate encourages you to take time to review these.