Compliance matters
We currently have a vacancy for a pharmaceutical assessor to join the Defective Medicines Report Centre (DMRC) team, working closely with the Inspectorate as part of the Inspection Enforcement and Standards Division of the MHRA.
The 2015 GMP inspection deficiency data trend has been published yesterday
In the October blog post we told you about the successful audit of the GMP Inspectorate under the JAP programme. Later that month the entire Inspectorate was assessed as part of the BEMA programme.
6 top tips for applicants submitting a Manufacturing Authorisation application or variation
The 2017 Orange and Green Guides are almost ready for publication.
Today sees the launch of the MHRA Blood Forum
The annual GCP Symposium was held by the MHRA on 20 September 2016 in Birmingham and was repeated on the 21 September 2016.
In October 2015 the MHRA was audited under the Joint Audit Programme (JAP) for EEA GMP Inspectorates.
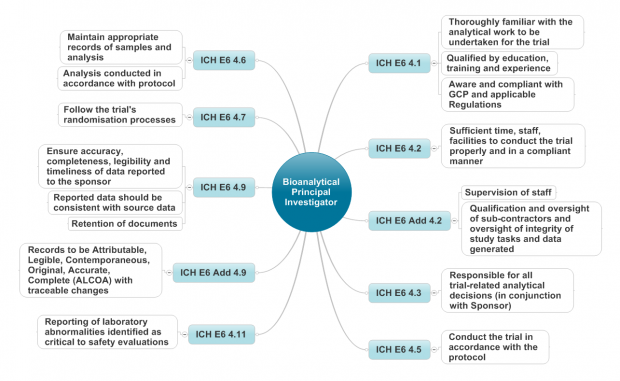
Earlier this year I represented the MHRA at the 10th Workshop on Recent Issues in Bioanalysis (WRIB).
When I applied to join the Inspectorate I was working as a Qualified Person. I’d been on the receiving end of many inspections and thought that the role looked to be a hugely interesting one with a great amount of …