Process Licensing Office joins the Inspectorate

The Process Licensing Office has today joined the Inspectorate to become the Inspectorate & Process Licensing Group.

The Process Licensing Office has today joined the Inspectorate to become the Inspectorate & Process Licensing Group.

This is the second in a set of three posts centred around the Responsible Person named on a Wholesale Distribution Authorisation (WDA). This post has been written to enhance the appointment of the RP.

This is the first in a set of three posts centered around the RP named on a WDA. This post provides information on external training recognition and training providers. The second of these three posts aim to enhance the appointment of the RP and the third post will place a particular emphasis on the engagement …
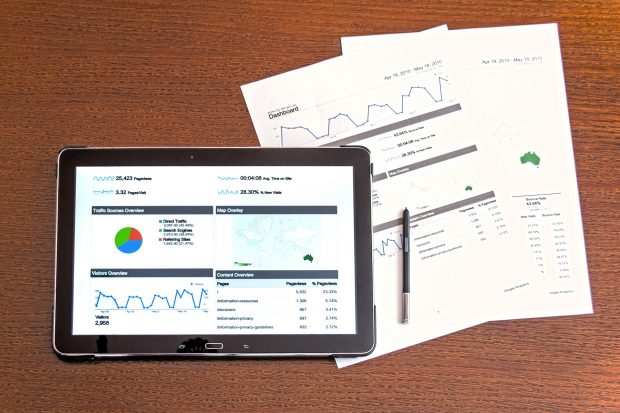
Guidance and examples of data integrity issues seen on GDP inspections.

From 16 to 18 April 2018 the PIC/S Committee and PIC/S Executive Bureau meetings took place in Geneva, Switzerland.

The GDP inspectorate has seen a growing number of false NHS and private prescriptions being used to obtain medicines from Marketing Authorisation Holders for the onward wholesale.
This post provides guidance around the urgent supply of unlicensed medicines that have needed to be imported out of hours by UK hospitals.

The MHRA’s GXP data integrity guide has been published today.

The focus of the 2017 GDP Symposium was risky business! This year the complimentary themes of Risk Management and Transportation ran throughout the various sessions at the event.

Whilst we progress our latest recruitment campaign for GMDP Inspectors, Ian Holloway reflects on 30 years of working for the Medicines Regulator.