Between 11 to 14 February 2020, the MHRA will be hosting a week-long series of events as part of the Good Practice Symposia Week. The week will include individual events from GPvP, GCP and GLP Inspectorates. Depending on your area of expertise, you can choose to attend the event most relevant for your needs or take advantage of discounted combined tickets. Find out more about each event below or visit https://mhra-good-practice-symposia.co.uk/home
GPVP Symposium (11 February 2020)
If you work in pharmacovigilance, medical information or regulatory affairs and want to hear from MHRA GPvP inspectors about the latest trends and topics in pharmacovigilance, then register for the GPvP Symposium 2020 today.
The event will include talks highlighting current challenges in pharmacovigilance faced by industry, the latest MHRA GPvP inspection metrics and an update on the GPvP inspection strategy. You’ll also have the chance to hear about post-marketing safety compliance metrics from the US-FDA and the cross-agency working initiatives between the two agencies focused on pharmacovigilance.
This year, the event will be made accessible to a wider audience through the livestream option, which includes the full morning programme.
For those choosing to attend the full day event, you will have the opportunity to join the afternoon technical sessions in the form of smaller seminars with inspectors.
As always, attending the symposium provides a great chance to share experience, network and speak to inspectors directly through inspectors’ surgeries and panel sessions. You’ll also be able to participate in the morning panel session through the livestream.
For more information, visit https://mhragpvp.co.uk/home
Keep connected: #MHRAGPvP20
Laboratories Symposium (12 February 2020)
We view our symposia as a vital part of our communication strategy, giving us an opportunity to engage with our stakeholders. The symposium will provide breakdowns of current laboratory regulatory requirements and expectations from monitoring authorities, as well as give attendees an insight into hot topics and areas of focus during regulatory inspections. Inspection metrics have been gathered from the annual inspection findings and will also be presented back to stakeholders. Questions may be submitted to the panel on the presentation topics and attendees can also drop in for one-to-one discussions at the inspector’s desk for any additional regulatory queries they may have.
The chosen theme for this year's laboratories symposium is: 'Fit for Intended Use'
This has been selected based on previous years stakeholder feedback, inspection metrics and hot topics. Presentations will include examples of the types of compliance issues facilities regularly have to deal with, as well as interactive walk-throughs of various scenarios. Presentations cover a range of related topics including:
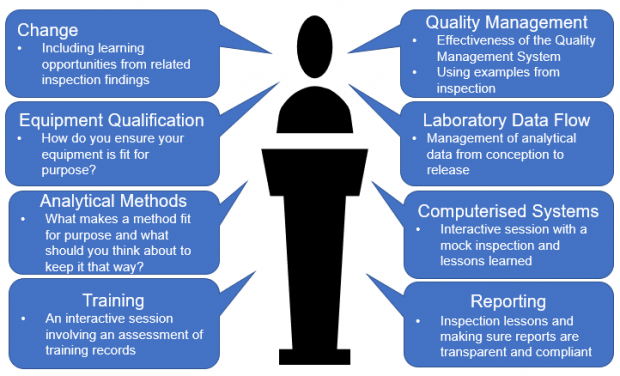
For even more information visit: https://mhralabs.co.uk/home
Keep an eye on all the news with #MHRALabs20
GCP Symposium (Commercial) 13 and 14 February 2020
This event is in partnership with the US Food and Drug Administration (FDA) so expect it to be another sell-out event. The full event will be two days, with interactive presentations on day one followed on day two with interactive workshops which will allow you the opportunity to work on relevant case studies. The agenda is primarily focussed at commercial organisations, as non-commercial sponsors and investigator sites had the unique opportunity to attend a symposium aimed exclusively at them in September 2019.
New this year, and in anticipation of a waiting list which is usual for our events, we have provided the option to live-stream day one of the event to enable all four corners of the world to join us virtually, watch presentations, participate in live polls and submit questions to speakers.
This event will provide regulatory perspectives from both the MHRA and FDA on the importance of sponsor oversight of clinical sites and laboratories, eSource including electronic health records, protocol deviations featuring the impact on data reliability as well as subject safety and the challenges in ensuring data quality in novel clinical trial designs. On day two, a variety of GCP and bioequivalence workshops will allow you to put your learnings into action.
As always, you will have the chance to put your questions directly to inspectors both from FDA and MHRA as well as having a chance to network and discuss queries during their inspectors’ surgeries.
For more information, visit https://mhragcp.co.uk/home
You can follow take home messages and highlights of the event using #MHRAGCP20
Don’t miss the next post, sign up to be notified by email when a new post comes out

