
The focus of the 2018 GDP symposium was maintaining compliance in a changing world. This year the complementary themes of change management and control ran throughout the various sessions at the event.
As one of the GDP operations managers, I took the lead for the planning and delivery of the GDP symposium on 19 and 21 November in London and 27 November in Glasgow and, once again, as a result of all of the hard work from those involved, these events were very well-received.
As with previous years, the London event sold out very quickly and a record number of delegates attended the event in Glasgow.
Richard Andrews (Unit Manager Inspectorate Operations GMP and GDP) started the GDP days with an opening presentation providing an update for delegates on changes at the agency - including the successful completion of our move from Victoria to Canary Wharf and the latest news on our Operational Transformation programme.
Richard also covered the changes to the Inspections, Enforcement and Standards (IE&S) divisional structure. Since the last symposium, the Process Licensing team has been brought into the same group as the Inspectorate, and we have welcomed a new GDP inspector to the team, Mariam Naqesh-Bandi.
Richard then moved on to provide an update on EU Exit. The event took place between the announcement of a draft withdrawal agreement and planned votes to accept or reject that agreement in the EU and UK parliaments, so in the days and hours running up to the event, the team kept a close eye on developments to ensure this session provided up to date information on the potential outcomes of the process. This was also an opportunity to bring delegates up to speed on the agency’s preparations for deal and no-deal scenarios.
Senior GDP Inspector Cheryl Blake then spoke about preparing for a changing world.
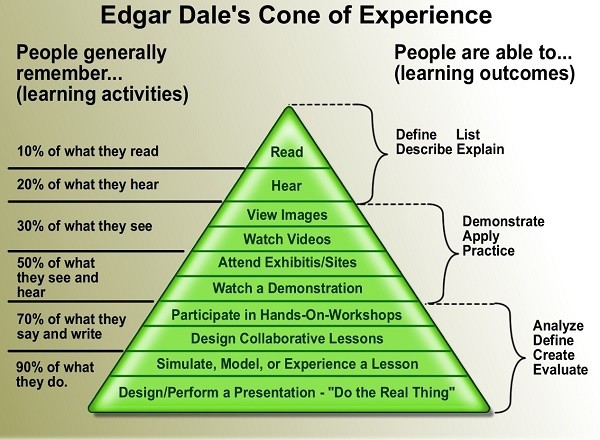
Cheryl was followed by Gaynor Brummitt (GDP inspector), who covered the topic of the Management of Change. Gaynor gave the delegates some important points to consider when risk-assessing change management and also provided some insights into how much of what we read is retained. Gaynor used Edgar Dale's Cone of Experience to demonstrate that people generally remember more through learning activities than other mediums. This is worth considering when conducting training and assessing staff understanding of the changes being made within your company.
Leading Senior GDP Inspector Tony Orme, and GDP Inspector Terry Madigan, provided delegates with some real examples of the cost of non-compliance to companies.
Their key take home message was:
failure to act to identify and rectify non-compliance has consequences for patients, your company and you.
The lunch break gave the delegates a further opportunity to network and speak informally with the inspectors. This was in addition to the ‘inspectors’ surgeries’ that were also in place throughout the day and allowed the delegates to speak with the presenters and discuss relevant topics. The large screens located within the foyer displayed recently-asked questions. Additional questions submitted through the event app during the day will be reviewed and posted into the delegate area of the app.
The break also gave delegates an opportunity to visit the various exhibitor stands.
The afternoon sessions got underway with GDP Inspector Peter Brown, who covered the popular topic of the top deficiencies identified during GDP inspections. Data was obtained from major and non-IAG referred critical findings. This data set reflects around 90% of UK distribution channels.
The top five deficiencies were reported as follows:
- Chapter 1 Quality Management – 38% of inspections
- Chapter 9 Transport – 25% of inspections
- Chapter 5 Qualification of Customers – 20% of inspections
- Chapter 6 Returned Medicinal Products – 18% of inspections
- Chapter 2 Responsible Person – 15% of inspections
The afternoon continued with Senior GDP Inspector Madeleine Ault, who presented the topic of keeping knowledge up to date in a changing business environment.
Madeleine covered key points surrounding personal learning action plans, namely:
- identification of role improvement opportunities
- evaluation of learning needs
- increasing knowledge of licence activities
- undertaking new learning opportunities
- maintaining learning records
Alan Bentley (GDP inspector) then took to the stage to provide delegates with an update to the requirements surrounding Active Pharmaceutical Ingredients (APIs) and when a registration is required.
|
Manufacture |
Do you manufacture Active Substances (AS)? |
Yes |
Are the AS for onward supply? |
No | Registration required: Manufacture | ||
| Yes | Registration required: Manufacture and Distribution | ||||||
|
Importation |
Do you procure AS from outside the EEA? |
Yes |
Are the AS for onward supply? |
No |
Registration required: Importation |
||
|
Yes |
Do AS enter the EEA? |
No |
Registration required: Distribution | ||||
|
Yes |
Registration required: Importation and Distribution | ||||||
|
Distribution |
Do you procure AS from within the EEA? |
Yes |
Are the AS for onward supply? | No | Registration required: none | ||
| Yes | Registration required: Distribution | ||||||
| Is your site used by another registered company to hold AS for a period of time? | No | Registration required: none | |||||
| Yes | Registration required: Distribution | ||||||
We were joined by Naseem Hudroge (Senior Intelligence Analyst) and Bruce Figg (Intelligence Analyst) of the MHRA’s Enforcement section. They provided a recap and update of the investigations surrounding the illegal diversion of Z drugs. They moved on to emerging trends and raised delegates’ awareness around the issues of Social Engineering, Phishing and email-spoofing.
Their key message:
Social engineering relies heavily on human interaction and psychological manipulation of people into breaking normal security procedures.
Licence holders should always remain vigilant.
The rapidly approaching 9 February 2019 deadline for implementing the ‘Safety Features’ element of the Falsified Medicines Directive provided an ideal opportunity for GDP Inspector Newaj Khan - together with Richard Andrews and Senior GDP Inspector Peter Blundell - to recap on the requirements, the recent consultation on the steps required for the UK to meet its obligations to transpose these provisions into UK law, and to review progress to date in implementing the European and National Medicines Verification Organisations (EMVO and SecurMed) and systems.
MHRA have set up a dedicated email address to deal with any questions and queries specifically relating to the Falsified Medicines Directive, which can be accessed at FMD.safetyfeatures@mhra.gov.uk
The day concluded with the ever-popular quiz, which enabled delegates to utilise the event app and to test their knowledge and key learning points from the day.
With GDP being such a large, complex and varied business to operate within, the aim of the event was to include something for everyone, whilst simultaneously delivering core messages in areas where it is felt more focus is required.
The inspectors enjoyed the opportunity of meeting with the delegates and were pleased to welcome back regular delegates, as well as those attending for the first time. The event app has been updated to include the presentations. We have received a substantial number of positive comments and feedback and are pleased that those who attended found the event useful and interesting. The output from the post evaluation survey will be used in the preparation of the 2019 event.
A huge thank you to all who attended: the presenters and organisers, the events management team, Glasgows, and our own Communications department – all of whom contributed to making the event such a success. We are already looking forward to welcoming you all to the 2019 event.
Don’t miss the next post, sign up to be notified by email when a new post comes out

