In March of this year, a letter was sent to Manufacturer’s “Specials” (MS) Licence holders who were involved in the manufacture of sterile products. An overview of unlicenced medicines guidance is provided here: Supply unlicensed medicinal products (specials)
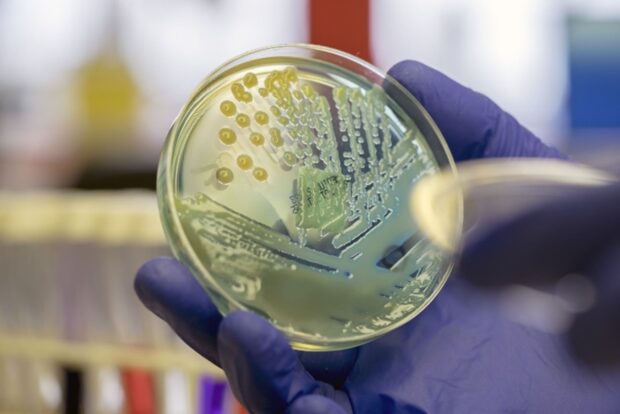
This was sent by the MHRA as part of the continued focus on the control of aseptic operations, linked to the expectations for the sanitisation of components and equipment being transferred into the grade A working zone, and also activities linked to the preparation of pooled bulk products (pooling) or intermediate products.
The letter was in response to the outcome of an inquest held at Coroner’s court that concluded in late 2024, with regards to events in 2014 linked to the supply of Total Parenteral Nutrition which was found to have been contaminated with a pathogenic microorganism.
The letter can be found here: MHRA Letter to MS Licence Holders
The focus within the letter reinforces the guidance defined in the current MHRA Guidance for Specials Manufacturers document, commonly known as the ‘Specials Q&A’. This document has been updated since the events of 2014 and sets out the standards for the manufacture of aseptically prepared products. This blog has been generated to support the extended reach of this letter so that the contents can be considered by these sites and also other parties, where appropriate to their current or planned future operations or via training.

