Several companies have contacted the MHRA regarding cannabis-based products for medicinal use. This blog provides information on what authorisations are required in order to manufacture within this sector of the pharmaceutical industry.
manufacture within this sector of the pharmaceutical industry.
For an overview of unlicensed Cannabis-Based Products for Medicinal use (unlicensed CBPMs), please see the following guidance which was updated in March 2020:
Licensing
Companies wishing to manufacture cannabis-based medicinal products or active pharmaceutical ingredients require authorisation by both MHRA and the Home Office.
Controlled Drugs Licence
- A Home Office issued Controlled Drugs (CD) licence is required for the cultivation, production, supply, and possession of cannabis. This requires an inspection by UK Home Office to ensure that appropriate procedures and facilities are in place to ensure the security of controlled substances.
Manufacturing Site Authorisations
- An MHRA issued manufacturing licence or API registration is required to manufacture CBPMs and active pharmaceutical ingredients (API). This requires an inspection of the site to confirm it has an appropriate facility, staff and Pharmaceutical Quality Management System and has carried out the required activities (including process and analytical validation).
Where a company has no existing authorisations in the UK
Historically, companies applying to manufacture and handle controlled drugs typically had existing MHRA licences to cover their manufacturing activities and could apply to the Home Office for authorisation to handle controlled drugs. An increasing number of companies with no previous pharmaceutical experience are entering the pharmaceutical sector solely to cultivate, process and manufacture cannabis-based products destined to be used as medicines. This results in a situation where a company needs to be authorised by both MHRA and the Home Office. This leads to a conundrum because:
- The MHRA require you to fully demonstrate your ability to consistently and correctly manufacture your product in order to grant an authorisation.
- Before issue of a controlled drugs licence, the Home Office need assurance that a company can meet the requirements of the MHRA and the licence is therefore necessary. So surely MHRA must go first?
BUT
- You can’t complete all the necessary process validation and performance qualification (PQ) activities required for the MHRA without handling the controlled substances and you can’t handle them until you hold a Home Office licence.

As this emerging sector of the pharmaceutical industry began to grow in the UK, discussions began between the MHRA and the Home Office to look at how we could provide a joined up approach to approving companies that would meet the need of industry whilst satisfying the regulatory needs of both government departments. As described above, there isn’t a way to operate both authorisation processes independently because each regulatory authorisation process requires some aspects of the other.
The following outlines the agreed approach to be followed for companies that do not already hold the necessary authorisations from either government department.
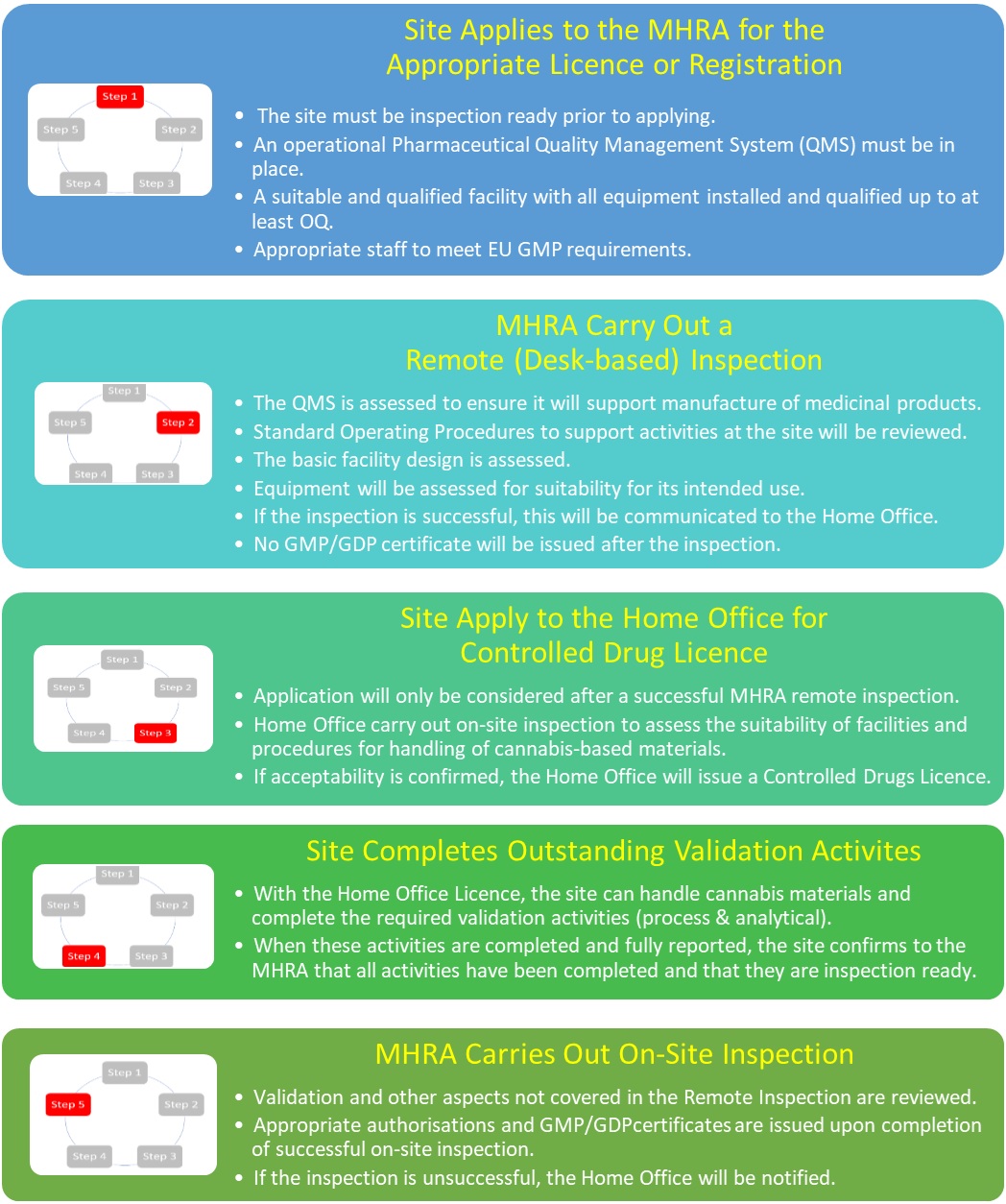
Only when appropriate authorisations have been granted by both MHRA and the Home Office can medicines be supplied.
Of the applications received to date, several companies have applied before they were fully ready to be inspected with the intent of completing the activities in the period between the application and the inspection.
At the point of application, you should have all necessary systems, equipment, and procedures in place. All qualification and validation of the facility and equipment should be complete up to the point of at least operational qualification (OQ). As stated above it is accepted that no process validation runs can be completed at the point of application as you will be unable to handle the controlled materials at that time, and that some PQ activities may also be incomplete. You may find this previous blog helpful: https://mhrainspectorate.blog.gov.uk/2017/01/05/helping-us-to-help-you/
If a company applies for a licence/registration before they are ready for inspection (e.g. the equipment is not in place or appropriately qualified) and this is identified during an assessment of the application, it will be rejected before commencing the initial remote inspection. If this is identified during the remote inspection, then the inspection will be ceased, the application will not be supported, and the company will be liable for the fees incurred up to that point. A further application will then need to be submitted.
Existing Manufacturing Sites
Where a site has an existing MHRA manufacturing licence or API registration, the Pharmaceutical Quality System in place will already have been assessed. Consequently, if the company wishes to handle cannabis-based materials, the Home Office may be able to process the application for a new, or revised, CD licence or the process already described may need to be followed. This will depend on how much the activities differ to those already authorised.
An MHRA inspection may be triggered when the variation to the site’s licence, or registration, is submitted, and this will be assessed on a case-by-case basis following our standard approach. Depending on the type of cannabis-based products to be handled, you may also need to amend the activities on your manufacturing authorisation or API registration.
New Importation Sites (Virtual)
Where a company is new and intends to be a virtual importation site (i.e. not directly physically handling cannabis-based materials by utilising a site that already has the appropriate MHRA and HO licences as the site of physical importation), they may still need a Home Office controlled drugs licence at that ‘virtual’ site in addition to authorisation from MHRA.
What type of authorisation do we need from MHRA?
There’s a simple answer to that question – it depends. To determine what authorisations are required we need quite a lot of information, including:
What is taking place where?
This is for all steps except cultivation. If all you want to do is grow cannabis plant, harvest it and sell it as an unprocessed crop, Good Manufacturing Practice isn’t applicable, and you are not within our remit. You need to comply with Good Agricultural and Collection Practice (GACP) which is an unlicensed activity. If you are performing any further processing steps (including drying and trimming) we need to know what is happening where. This also includes steps AFTER you have sold your product, because the subsequent activities carried out can change what type of licence you need.
What is going to happen to the material you are putting into the supply chain?
What someone else does with the material you provide to them can change what approvals are required. The point at which a material becomes an active pharmaceutical ingredient, or a medicine can differ depending upon what further processing steps there are. For example, cannabis flower/flos/bud could be a starting material, an active pharmaceutical ingredient, or a finished product (i.e. a medicine) so your position in the manufacturing process is dependent on what happens next and not just what you do. This is because the regulatory framework sits around the product, not the process - so it is important that you understand where you, your activities, and your product fit relative to the finished medicine that the patient will receive.
Summary and What Next
We hope this overview helps you understand the regulatory process currently in place for these types of products, and provides some context to some of the challenges around authorisations related to cannabis-based products for medicinal use.
We intend to publish a further blog in the coming months which will help you determine if your activities would require an application for an API registration, a manufacturing licence, or both.

