My name is Graham Carroll and in August 2017, I took over from Michelle Yeomans (formerly Rowson) as one of the three GMDP Operations Managers. As part of this I inherited overall responsibility for planning and delivery of the GMP Symposium. I am pleased that once again we delivered a professional and well-received event, and I can share some of the highlights with you here.
As in previous years we made use of the MHRA Event App, enabling delegates to access presentation materials and to participate in the interactive sessions using their own electronic devices. For the first time, there were ‘Lunch and Learn’ videos played during the lunchtime break, giving delegates a greater choice in how to use their time during the event. Over the last few years several highly knowledgeable and experienced professionals have joined the Inspectorate, and this year some of them took to the stage for the first time.
The GMP day started with an opening presentation by Richard Andrews (Unit Manager Inspectorate Operations GMP and GDP), who provided an update on Agency, International and Inspectorate activities. This included updates on the planned move of our headquarters from Buckingham Palace Road to Canary Wharf, the Operational Transformation programme and Brexit. As anticipated, we received several questions throughout the event about Brexit; all the latest information will continue to appear on our website and Richard re-iterated that the Agency is working closely with the government to analyse the best options and opportunities available for the safe and effective regulation of medicines and medical devices in the UK. While negotiations continue the UK remains a full and active member of the EU, with all the rights and obligations of EU membership firmly in place.
The first set of presentations set the scene for the day and covered some of the areas of organisational processes and culture that can impact on risk management. Vivian Rowland (GMDP Inspector) started with her session ‘Quality Risk Management – Why is it important and have you got it right?’. Beginning with the basics of understanding risk, Vivian moved on to reviewing how regulatory guidance has changed over the years to embrace the concept of risk management; the 2002 ‘Orange Guide’ had only 30 references to risk, whereas the current guidance has over 230. Vivian then explored some relevant deficiencies from recent inspections so that delegates could look for similar weaknesses in their own systems. Vivian’s presentation finished with a pertinent quote from Tom Gilb:
“If you don’t actively attack the risks, they will actively attack you!”
David Churchward (Expert GMDP Inspector) and Bob Wheeler (The Wheeler Partnership) delivered an engaging and thought-provoking session on ‘Critical Thinking and Risk Management’. Bob and David demonstrated how easy it can be to jump to conclusions, and used real-world examples of when a lack of critical thinking has led to disaster. Using interactive tools, delegates took part in a case study to encourage critical thinking, reviewing a Product Quality Review (PQR) and coming up with additional questions they would want to ask to supplement the information presented.

Ewan Norton (Senior GMDP Inspector) was up next, discussing ‘Organisational Culture and Knowledge Management’. Ewan’s presentations are always popular and this year he enrolled the help of his family in a film giving a humorous take on a company’s preparations for an inspection, coming up with as many delaying tactics as possible; however, these were all real examples from MHRA inspections! The attitude and behaviour of senior management in these situations has unfortunately been seen to ‘trickle down’ to the shop floor resulting in poor compliance, sometimes to the surprise of the very same managers. Ewan then gave some insights into the need for effective knowledge management; making it easy to add and retrieve information is critical to an effective knowledge management system. Ewan ended with a poignant reminder that linked back to David and Bob’s case study, highlighting that in fact this was a real-world example where patients had received substandard medicines due to a lack of critical thinking and effective knowledge management.
After a break for refreshments and a chance to network with other delegates, the event resumed with several sessions covering the application of Quality Risk Management to the production environment. This was prefaced by a brief regulatory update from Martine Powell (GMDP Inspector) focussing on those updates which were most relevant to production.
 Andrew Hopkins (Expert GMDP Inspector) delivered a session updating the audience on progress towards publication of an update to Annex 1 (Sterile Medicinal Products). At the time of the Symposium, publication of the consultation document was imminent. Andrew shared the proposed text from several sections, and showed that the principles of Quality Risk Management would be embedded throughout the updated text. Andrew went on to demonstrate how Quality Risk Management principles can be used to reduce risks to sterility assurance, using the example of a powder dosing system design which significantly reduced the number of aseptic manipulations for one manufacturer. I am pleased to say the Annex 1 consultation has now been published, and the consultation period will run until 20 March 2018.
Andrew Hopkins (Expert GMDP Inspector) delivered a session updating the audience on progress towards publication of an update to Annex 1 (Sterile Medicinal Products). At the time of the Symposium, publication of the consultation document was imminent. Andrew shared the proposed text from several sections, and showed that the principles of Quality Risk Management would be embedded throughout the updated text. Andrew went on to demonstrate how Quality Risk Management principles can be used to reduce risks to sterility assurance, using the example of a powder dosing system design which significantly reduced the number of aseptic manipulations for one manufacturer. I am pleased to say the Annex 1 consultation has now been published, and the consultation period will run until 20 March 2018.
The next session was delivered by Graeme McKilligan (Leading Senior GMDP Inspector) and Philip Rose (GMDP Inspector), on the topic of ‘Cross Contamination, QRM approach and examples of application of control measures’. Cross contamination, health based exposure limits and permitted daily exposure have been discussed in some depth at previous GMP symposia, so this session focussed mainly on organisational controls to minimise the risk of cross contamination. Graeme explained how important it is to observe and understand the process in practice, measure the current performance, and then using risk management tools to analyse, evaluate and improve this performance to reduce the risk. Philip then built on several elements of an example risk assessment to show how additional organisational control measures could be developed for the specific risks. In closing, Philip and Graeme reminded delegates of the importance of ongoing risk reviews.
The final presentation of the morning session was a session on ‘Data Integrity Risk Assessments (DIRA) and considerations for control systems’. Tracy Moore (GMDP Operations Manager and Senior GMDP Inspector) is the GMP Inspectorate’s lead on Data Integrity, and she was supported by Kevin Bailey (GMDP Inspector) who joined the team in 2016 and has a background in Computerised System Validation. Industry is becoming more automated and, as a result, systems and data flows are becoming more complex. Tracy and Kevin addressed some common myths about data integrity risk assessments, explored some example deficiencies, and then set delegates to work on a range of scenario questions. Delegates were challenged to identify key risk assessment considerations for data integrity in each scenario. Tracy finished by providing an update on the GXP Data Integrity Guidance consultation and also highlighted that future Data Integrity blogs were in the pipeline; watch this space!
After a long morning, a welcome break for lunch gave delegates another opportunity to catch up with peers and chat to inspectors. As mentioned earlier, three ‘Lunch and Learn’ videos were shown in the main conference area during the lunchtime break, covering a range of topics:
First, Richard Parker (Senior GMDP Inspector) spoke about the licensing requirements for the manufacture of veterinary medicines. Richard’s expertise as a result of his prior experience as a GMP Inspector with the Veterinary Medicines Directorate (VMD) has been a very welcome addition to the MHRA. Richard explained the different authorisation types, as well as the associated requirements for inspections and GMP certificates.
Next up, Alan Moon (Senior GMDP Inspector) gave an update on Investigational Medicinal Products (IMP). This included regulatory changes and timeframes, common queries on IMP manufacture, and a reminder that any transitional IMP QPs in the UK who have not yet submitted reassessment applications to MHRA should do so to avoid the risk of not being able to be nominated to be named on a different MIA(IMP) after implementation of the Clinical Trials Regulation.
Lastly, Richard Andrews returned (on screen at least) to provide insights into the expectations for Active Pharmaceutical Ingredient (API) audits in order to fulfil the requirements for QP declarations in Marketing Authorisation applications and variations. This video was intended to assist licence holders in making valid QP declarations, and to avoid questions during Marketing Authorisation assessments.
The event resumed for the afternoon with the focus shifting to the application of Quality Risk Management to the supply chain. Again, Martine Powell gave a regulatory update looking at those areas which were most relevant to the supply chain. With the forthcoming implementation of the FMD ‘safety features’ in February 2019, drafting of Annex 21 ‘GMP for Importers of Medicinal Products’, new and updated Mutual Recognition Agreements (MRAs) with Canada, Japan and the USA, there was a lot for delegates to keep themselves appraised of. Martine also gave an update on the work of the International Coalition of Medicines Regulatory Authorities (ICMRA).
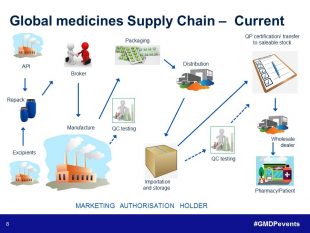 The last presentation of the day was another team effort. Norman Gray (Senior GMDP Inspector) and Ian White (GMDP Inspector) came together to share their experiences in their session ‘Supply Chains – What can possibly go wrong’. They were supported on-screen by Ian Holloway (Senior GMDP Inspector) who had recorded a video looking back on 30 years with the medicines regulator in its various guises. From this overview of the simple supply chains of the past, Norman demonstrated how much more complex the pharmaceutical supply chain is now, using an example where six sites in four countries are involved in the manufacture, packaging, storage, testing and release of a biological product. Norman and Ian then went on to run through four different case studies focusing on various aspects of the supply chain including manufacture, transportation, and testing, and showed how risk management principles could be applied in each case.
The last presentation of the day was another team effort. Norman Gray (Senior GMDP Inspector) and Ian White (GMDP Inspector) came together to share their experiences in their session ‘Supply Chains – What can possibly go wrong’. They were supported on-screen by Ian Holloway (Senior GMDP Inspector) who had recorded a video looking back on 30 years with the medicines regulator in its various guises. From this overview of the simple supply chains of the past, Norman demonstrated how much more complex the pharmaceutical supply chain is now, using an example where six sites in four countries are involved in the manufacture, packaging, storage, testing and release of a biological product. Norman and Ian then went on to run through four different case studies focusing on various aspects of the supply chain including manufacture, transportation, and testing, and showed how risk management principles could be applied in each case.
After a quick break for refreshments, we were back in the room for the ever popular ‘Q&As to the MHRA panel’. Technological advances this year meant that as well as submitting questions using the MHRA app, delegates could also see questions that had been submitted by others and vote for those that they most wanted to see answered. This meant that we could easily identify the most popular questions and ensure that our panel of Inspectors answered as many of these as possible. The Inspectorate commit to providing a written response to all questions submitted and the Q&As will be collated and made available to delegates within the next few months.
The event was drawn to a close by Richard Andrews who reminded delegates of the key themes of the day and offered thanks to the delegates, speakers and various support teams who make the event possible.
We hope the event proved useful for delegates and that everyone went home with one or more learning points to apply. The resources pages of the GMDP Symposium website and app have now been updated to include all presentation content as well as the transcripts of the Lunch and Learn videos. As always, we are keen to continue evolving the format and content of the symposium to enhance stakeholder engagement. The post evaluation survey is open to delegates, but we are also happy to receive comments in response to this blog to help us plan for next year’s event.
Don’t miss the next post, sign up to be notified by email when a new post is published on the Inspectorate blog.


2 comments
Comment by Anne Le Bris posted on
Please receive all my thanks for sharing openly information related to GxP.
I believe that transparency is key in helping all actors deeply involved in building an effective Health Industry for the benefit of patients. Anne
Comment by beiweiwudu posted on
I appreciate what you have done . but I want to know whether the resources of GMDP symposium is free or limited? where and how can I get these ducuments? Thank you!